About the Course
This eLearning module is designed to provide a comprehensive understanding of Pharmaceutical Risk Management, with a focus on the application of Quality Risk Management (QRM) principles in the pharmaceutical industry. The training is structured to cover key aspects of risk management, from foundational concepts to advanced tools and real-world applications. It aims to equip learners with the knowledge and skills necessary to eDectively assess the implementation of QRM principles within pharmaceutical operations.
Pharmaceutical Risk Management
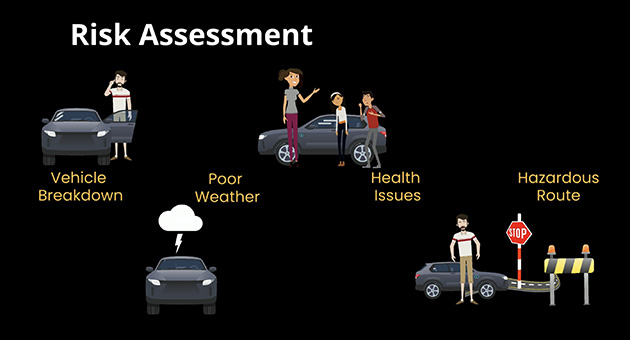
- Risk Assessment in Pharma Industry
- Inclusion of Quality Risk Management (QRM) in Schedule M, Revision Dec. 20223
- Concept of Quality Risk Management and its components
- Understanding Formality, Uncertainty, Importance, and Complexity
- Risk question/problem definition
- Risk assessment involving determining probability of a risk, and its potential severity, and based on that prioritizing the risk mitigation efforts.
- Risk Control/Mitigation including risk reduction measures, and the acceptance of remaining risk, if any
- Risk Review
- Responsibility of Management when handling Risks
- Risk Management Tools
- Basic Methods like flow charts, check sheets, process mapping, cause & effect diagram
- Advanced Methods like risk matrix, Failure Mode Effects Analysis (FMEA), Failure Mode Effects and Criticality Analysis (FMECA), Fault Tree Analysis (FTA), Hazard Analysis (HACCP, HAZOP, PHA) and other supporting tools.
- Risk Reduction Measures: prevention and protection
- QRM integration in quality systems
- Adoption of QRM Principles
- Specific adoption for Biological Products
- QRM Application & Misuse
- Case study: On supplier auditing frequence
- Commonly observed challenges in QRM Application
- Global Regulatory Observations Related to QRM
Summary and Conclusion: Intent of QRM
Final Evaluation: 3 attempts to achieve 70% in the evaluation.
Earn a career certificate

-
Lesson - Pharmaceutical Risk Management - Schedule M
Video
-
Check Your Knowledge
Assessment
-
Discussion Forum
Discussion Forum
-
Feedback
Feedback
-
Certificate
CDSCO Certificate
Course Rating
1 Review
-
100%
-
0%
-
0%
-
0%
-
0%
-
Rating User
10-09-2024
Test 2